Technique Used to Separate Liquids From One Another
It can be used to obtain a product that. Chromatography is used to separate mixtures of coloured compounds.
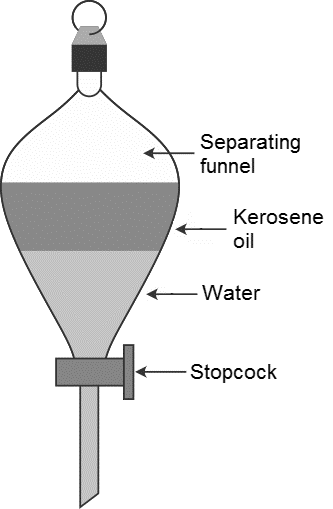
Name The Method You Shall Use To Separate Two Immiscible Class 11 Chemistry Cbse
The two liquids are put into the funnel and are left for a short time to settle out and form two layers.

. The two liquids are now separate. Chromatography involves the sample being dissolved in a particular solvent called mobile phase. The changing of a solid directly into vapours on heating and of vapours into solid on cooling is called sublimation.
Up to 24 cash back A separation funnel is a piece of laboratory equipment used with liquid extractions to separate a mixture into two solvent bases. - Leaves desired compound in its. Fractional distillation is carried out by using a fractionating column.
It was introduced by a Russian Scientist Michael Tswett. The change of a liquid to a gas to remove solvent and collect solute is called. Next to froth flotation the most useful foam separation technique is precipitate flotation where the species to be separated is first precipitated usually.
Distillation is an effective method to separate mixtures comprised of two or more pure liquids. This is a more common method of separating an insoluble solid from a liquid. The tap of the funnel is opened and the bottom liquid is allowed to run.
Used to separate solid or liquid substances from liquids by spinning at high speeds. Separating immiscible liquids is done simply using a separating funnel. A mixture of two miscible liquids can be separated by the process of fractional distillation.
When you pour the liquid off the top when sediment has settled to the bottom of the container. This technique is used to separate an insoluble solid from a liquid. The funnel is opened and as soon as the denser liquid has been filtered the tap is closed.
From the solid that sublimes upon heating from another substance with a high melting point. The most basic method would be distillation or boiling one of the liquids and collecting the condensation. Leaves impurities in their starting layer Choose.
Separation techniques froth flotation employs a relatively high gas flow-rate under turbulent conditions. It is used as a separation technique for mixtures which contain sublimable volatile substances and non-sublimable volatile components. Below are some common separation methods.
Similar to simple distillation fractional distillation is best for separating a solution of two miscible liquids. Some substances such as ammonium chloride camphor naphthalene and anthracene are sublime substances. Fractional distillation is a technique used to separate liquids according to their boiling points.
Oil and water can be easily separated using this technique. Washes and extractions are both techniques that use a separatory funnel to separate liquid layers. Liquids can be described in two ways immiscible and miscible.
This method is often used in the food industry. This is the technique used to separate a mixture of two or more miscible liquids with different boiling points. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize.
Chromatography is a separation technique used to separate the different components in a liquid mixture. Leaves impurities in their starting layer choose. The separation technique used for each liquid depends on the properties of the liquids.
This means that the two separate bases have different densities. Chromatography is vast separation technique which has many. Moves impurities from one layer to another choose.
This is usually accomplished with a perforated barrier wire screen non-woven fiber or granular media that allows the liquid to pass but retains the solids. Distillation is a purification process where the components of a liquid mixture are vaporized and then. Washes and extractions are both techniques that use a separatory funnel to separate liquid layers.
Thin-layer chromatography is a special type of chromatography used for separating and identifying mixtures that are or can be colored especially pigments. However washes and extractions have differences Determine whether each statement applies to washes or extractions. Distillation can be used to separate solutions of miscible liquids because the liquids have different boiling points.
Sublimation is the process in which a substance directly changes from a solid state to a liquid state. Decantation can be used to separate two liquids that have different densities as long as they are immiscible. It really depends on which two liquids you are try to separate.
The mobile phase may be a. Separating funnel is used mainly to segregate two immiscible liquids. It uses distillation to fractionate.
Water being denser settles at the bottom and oil floats on water forming two distinct layers. Alcohol and water are miscible liquids. Choose Watch Leaves desired.
One way to separate a soluble solid from its solution is to make crystals. This technique is also takes quite a bit of time because you have to wait for the mixture to separate which depending on it can take up to hours. However washes and extractions have differences Determine whether each statement applies to washes or extractions Moves desired compound from one layer to another Choose.
The separation of two liquids by fractional distillation depends on the difference in their boiling points. The mixture is dissolved in a fluid called the mobilephasewhich carries it through a structure holding another material called the stationaryphase. For example water and oil form two separate layers when mixed together.
The next most common is gravitational density separation where the solids float and are mechanically skimmed or removed by a spillway or sink and the liquid is removed by a spillway. The mechanism involves taking advantage of the unequal density of the particles in the mixture. When one substance in the mixture has some magnetic properties then this method is quite useful.
The process of sublimation is used to separate those substances from a mixture that sublime on heating. Used to separate two liquids that cannot dissolve in one another immiscible liquid separates two liquids by taking advantage of unequal mass. What is the separating funnel technique.
Up to 24 cash back Used to separate a liquid from an insoluble solid. Chromatography is a separation technique used to separate the different components in a liquid mixture. Distillation works because it.
This difference in the solubility of sugar and sand in water is used to separate them. Substances are separated based on their weight.

What Points Do We Use To Separate Two Liquids Quora

Separation Of A Mixture Worksheet And Lab Collection Matter Unit Separation Paper Chromatography

0 Response to "Technique Used to Separate Liquids From One Another"
Post a Comment